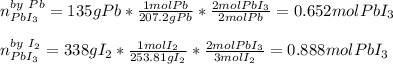Chemistry, 27.01.2021 06:50 Theresab2021
Calculate the mass of lead (III) iodide, Pb13, that can be produced by the reaction of 135 g of lead, Pb,
and 338 g of iodine.
1. What is the limiting reactant?
2. How much product is formed?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, angelinadhar
What are the percent by mass of copper in penny lab
Answers: 3

Chemistry, 22.06.2019 03:00, Dkhaurithompson
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

You know the right answer?
Calculate the mass of lead (III) iodide, Pb13, that can be produced by the reaction of 135 g of lead...
Questions in other subjects:





Mathematics, 16.04.2020 20:48


Mathematics, 16.04.2020 20:48



Mathematics, 16.04.2020 20:48