
Chemistry, 27.01.2021 04:00 anonymous1813
Using the model for the formation of Scandium Fluoride (an ionic compound), write the chemical formula.
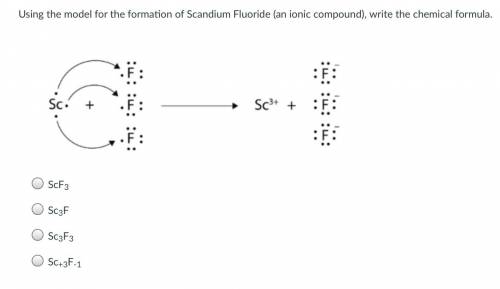

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:40, trinityanne1738
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 19:10, aamu15
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3


Chemistry, 23.06.2019 00:30, vane6176
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3
You know the right answer?
Using the model for the formation of Scandium Fluoride (an ionic compound), write the chemical formu...
Questions in other subjects:


Business, 21.07.2019 18:00


English, 21.07.2019 18:00




Mathematics, 21.07.2019 18:00


History, 21.07.2019 18:00



