
Chemistry, 26.01.2021 22:40 CutiePie8960
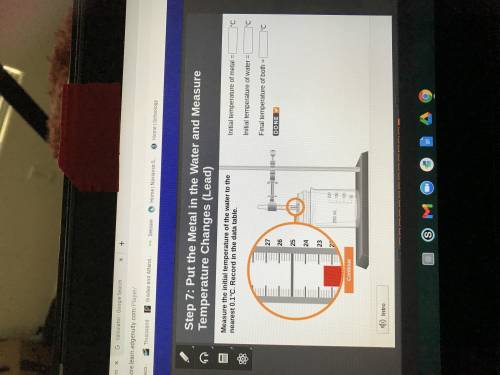
The temperature at 300 seconds (5 minutes) is the
final temperature of both the metal and the water.
Record it to the nearest 0.1°C in the data table.
Initial temperature of metal =
Initial temperature of water = 0
Final temperature of both =
'c
RETRY
250 ml
Continue (60s more)
05:00

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, Queenquestion5967
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3

Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 10:30, kylemartinez13
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2
You know the right answer?
The temperature at 300 seconds (5 minutes) is the
final temperature of both the metal and the water...
Questions in other subjects:



Mathematics, 28.07.2020 23:01

English, 28.07.2020 23:01








