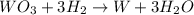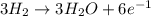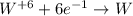Chemistry, 30.01.2020 02:59 atenagueroprivate
1. which of the following chemical reactions is an oxidation-reduction reaction?
answer
wo3 + 3h2 w + 3h2o
kno3 + licl lino3 + kcl
caso4 + 2nacl na2so4 + cacl2
mg(no3)2 + 2hbr mgbr2 + 2hno3
2. according to the collision theory and model created to explain the collision theory, what is the best explanation for why a higher concentration makes a reaction go faster?
there are more collisions per minute.
it increases the distance between the particles.
it increases the average kinetic energy of the particles.
it increases the average kinetic energy and there are more collision per minute

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, britotellerialuis
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 04:30, aleilyg2005
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 14:10, cameronbeaugh
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1
You know the right answer?
1. which of the following chemical reactions is an oxidation-reduction reaction?
answer
...
answer
...
Questions in other subjects:

Chemistry, 20.10.2021 20:50

English, 20.10.2021 20:50

Biology, 20.10.2021 20:50


History, 20.10.2021 20:50

Biology, 20.10.2021 20:50



Mathematics, 20.10.2021 20:50

Social Studies, 20.10.2021 20:50