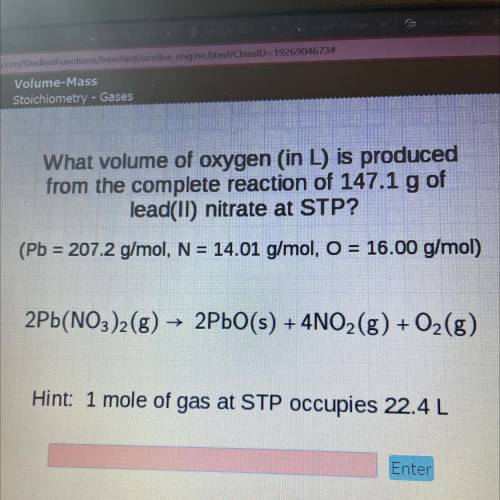
Lus
What volume of oxygen (in L) is produced
from the complete reaction of 147.1 g of
l...


Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, rscvsdfsrysas1857
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 23.06.2019 02:10, sativataurus
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2

Chemistry, 23.06.2019 07:00, asims13
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 18.07.2019 08:00

Mathematics, 18.07.2019 08:00

Physics, 18.07.2019 08:00

Biology, 18.07.2019 08:00

Mathematics, 18.07.2019 08:00

History, 18.07.2019 08:00

Mathematics, 18.07.2019 08:00


English, 18.07.2019 08:00

Social Studies, 18.07.2019 08:00