
The following reaction shows the products when sulfuric acid and aluminum hydroxide react.
2Al(OH)3 + 3H2SO4 → Al2(SO4)3 + 6H2O
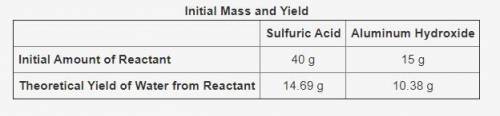
The table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory.
What is the approximate amount of the leftover reactant?
11.73 g of sulfuric acid
10.33 g of sulfuric acid
11.12 g of aluminum hydroxide
13.67 g of aluminum hydroxide

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, madlenserlipepu1o
__ _ _ _ _ is the process of removing earth materials from their original sites through weathering and transport and depositing the in another location. a. erosion b. sedimentation c. lithification d. dissolution
Answers: 1


Chemistry, 22.06.2019 05:30, mandy9386
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 10:10, jojomgarcia01
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2
You know the right answer?
The following reaction shows the products when sulfuric acid and aluminum hydroxide react.
2Al(OH)...
Questions in other subjects:



Mathematics, 22.05.2020 14:58

Mathematics, 22.05.2020 14:58



Mathematics, 22.05.2020 14:58

Biology, 22.05.2020 14:58

History, 22.05.2020 14:58

Computers and Technology, 22.05.2020 14:58



