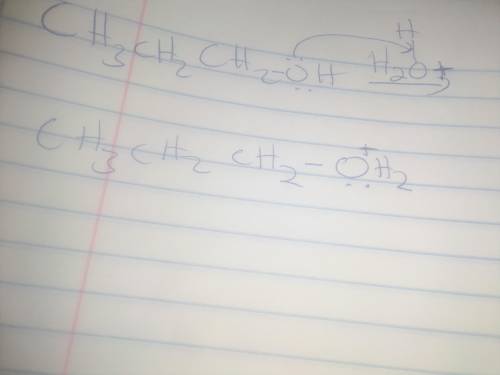When propyl alcohol is treated with acid, the initially formed intermediate is known as an oxonium ion. There is a scheme of a reversible chemical reaction. The substrates are CH3CH2CH2OH molecule and H with a charge of 1 plus ion. The product is CH3CH2CH2OH2 with a charge of 1 plus ion. Oxygen atom in CH3CH2CH2OH molecule has 2 lone pairs. Oxygen atom in CH3CH2CH2OH2 with a charge of 1 plus ion has a lone pair. All bonds are single. Using the curved arrow formalism, show how this process most likely occurs.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 07:30, superfly903
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2

Chemistry, 22.06.2019 14:30, Cartucho1978
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1
You know the right answer?
When propyl alcohol is treated with acid, the initially formed intermediate is known as an oxonium i...
Questions in other subjects:


Mathematics, 04.05.2021 03:10

History, 04.05.2021 03:10

Computers and Technology, 04.05.2021 03:10

Spanish, 04.05.2021 03:10



Mathematics, 04.05.2021 03:10

Mathematics, 04.05.2021 03:10