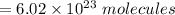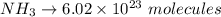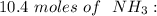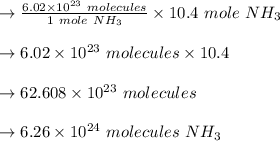Chemistry, 26.01.2021 02:30 dbhuggybearow6jng
How many molecules of ammonia are contained in 10.4 moles of ammonia, NH3?
O 0.611 molecules NH3
O 6.26 x 1024 molecules NH3
O 1.71 x 10-23 molecules NH3
O 177 molecules NH3

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 08:00, tchase0616
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1
You know the right answer?
How many molecules of ammonia are contained in 10.4 moles of ammonia, NH3?
O 0.611 molecules NH3
Questions in other subjects:

Biology, 05.05.2021 21:50


Chemistry, 05.05.2021 21:50



Health, 05.05.2021 21:50



Mathematics, 05.05.2021 21:50

Mathematics, 05.05.2021 21:50

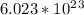 particles of the substance.
particles of the substance.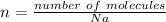
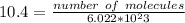
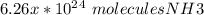 .
.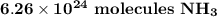 "
"