
Chemistry, 13.10.2019 07:50 raulriquelmef6p0947w
How do ionic bonds differ from covalent bonds?
a. electrons are transferred in a covalent bond.
b. protons are transferred in a covalent bond.
c. electrons are shared in a covalent bond.
d. protons are shared in a covalent bond.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:20, sarinaneedshelp01
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 14:40, neonbluefaith
Which statement best describes the function of enzymes?
Answers: 1

Chemistry, 22.06.2019 23:00, SophieCasey
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 22.06.2019 23:00, hailey5campbelp7d1c0
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2
You know the right answer?
How do ionic bonds differ from covalent bonds?
a. electrons are transferred in a covalent bo...
a. electrons are transferred in a covalent bo...
Questions in other subjects:




Chemistry, 24.06.2019 05:00



Social Studies, 24.06.2019 05:00

Mathematics, 24.06.2019 05:00


History, 24.06.2019 05:00

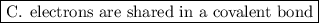 .
.


