
Chemistry, 25.01.2021 21:40 DESIRE44030
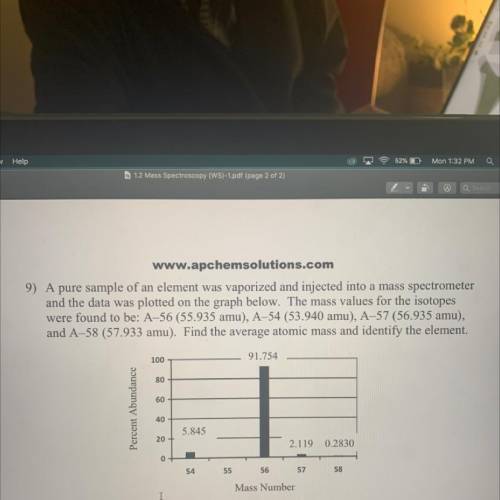
9) A pure sample of an element was vaporized and injected into a mass spectrometer
and the data was plotted on the graph belovr. The mass values for the isotopes were
found to be: A-56 (55.935 amu), A-54 (53.940 amu), A-57 (56.935 amu), and A-58
(57.933 amu). Find the average atomic mass and identify the element.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:10, sangamlama
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 21:20, skyemichellec
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
9) A pure sample of an element was vaporized and injected into a mass spectrometer
and the data was...
Questions in other subjects:


English, 02.09.2020 20:01

Mathematics, 02.09.2020 20:01


Mathematics, 02.09.2020 20:01

Mathematics, 02.09.2020 20:01



History, 02.09.2020 20:01

Geography, 02.09.2020 20:01



