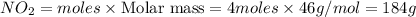Chemistry, 24.01.2021 23:20 jaksenpounders
Calculate the number of gams of nitrogen dioxide that are produced from 4 moles of nitric oxide
2NO(g) + O2(g) → 2NO2(g)
PLZ ANSWERRR FAST

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:30, MJyoungboy
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

Chemistry, 22.06.2019 22:30, kiera2599
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1


Chemistry, 23.06.2019 00:00, familyk0jj3
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
Calculate the number of gams of nitrogen dioxide that are produced from 4 moles of nitric oxide
2NO...
Questions in other subjects:

History, 18.10.2020 14:01


Mathematics, 18.10.2020 14:01


Mathematics, 18.10.2020 14:01


English, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01

Physics, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01

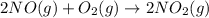
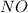 produce = 2 moles of
produce = 2 moles of 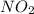
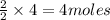 of
of