
Chemistry, 24.01.2021 22:20 mlguerrero12
Zn + 2HCI -> ZnCl2 + H2
a. How many moles of ZnCl, can be made from 1.7g of Zn?
b. If 6.4 grams of HCl are used in this reaction, how many moles of ZnCl, are
produced?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:10, nasrul3725
Remember to use the proper number of significant figures and leading zeros in all calculations. gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 11:50, robert7248
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 12:30, kaliyab191
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 13:00, yaneiryx5476
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1
You know the right answer?
Zn + 2HCI -> ZnCl2 + H2
a. How many moles of ZnCl, can be made from 1.7g of Zn?
b. I...
b. I...
Questions in other subjects:

Mathematics, 04.03.2021 22:40

Social Studies, 04.03.2021 22:40








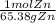 = 0.0273784032 moles Zn
= 0.0273784032 moles Zn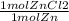 = 0.0273784032 moles ZnCl2
= 0.0273784032 moles ZnCl2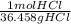 = 0.1755444621 mol HCl
= 0.1755444621 mol HCl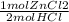 = 0.877722311 moles ZnCl2 are produced.
= 0.877722311 moles ZnCl2 are produced.


