Chemistry, 30.01.2020 14:54 LikeIke7105
Enough of a monoprotic acid is dissolved in water to produce a 0.0136 m solution. the ph of the resulting solution is 2.45. calculate the ka for the acid.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, kkelley9223
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2



Chemistry, 23.06.2019 04:31, cassiuspricerules
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2
You know the right answer?
Enough of a monoprotic acid is dissolved in water to produce a 0.0136 m solution. the ph of the resu...
Questions in other subjects:

SAT, 11.11.2020 04:30

Mathematics, 11.11.2020 04:30



Mathematics, 11.11.2020 04:30

World Languages, 11.11.2020 04:30

Mathematics, 11.11.2020 04:30


English, 11.11.2020 04:30

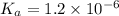
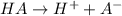



![[H^+]=c\alpha](/tpl/images/0486/2169/21a04.png)
![pH=-log[H^+]=2.45](/tpl/images/0486/2169/8dd78.png)