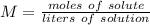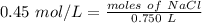1. How many moles of NaCl would be contained in .750 L solution with a molarity of
0.45M?...


Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, hannacarroll2539
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1


Chemistry, 23.06.2019 02:40, towelmearowel
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3

You know the right answer?
Questions in other subjects:

Mathematics, 01.10.2019 07:20




English, 01.10.2019 07:20



Mathematics, 01.10.2019 07:20

Physics, 01.10.2019 07:20