AL(S03)2 + Cu

Chemistry, 23.01.2021 14:00 adamgala3885
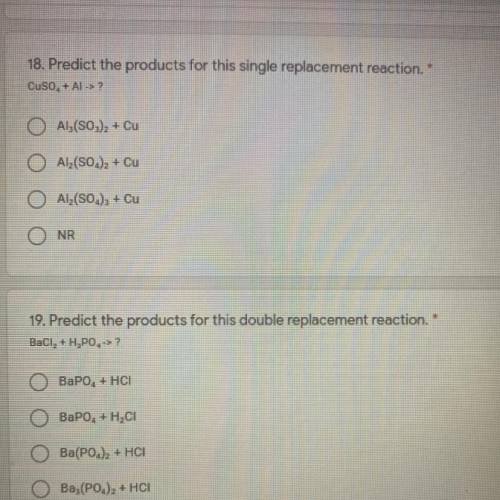
18. Predict the products for this single replacement reaction.
Cuso, + ALS
AL(S03)2 + Cu
Al(S0.)2 + Cu
O Al(SO.), + Cu
NR
19. Predict the products for this double replacement reaction.
Baci, + H, PO->?
BaPO4 + HCI
BaPO. + HCl
Ba(POJZ + HCI
Ba,(PO.)2 + HCI
PLEASE HELP ITS FOR A TEST I WOULD APPRECIATE IT !!

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, daniel1480
Problem page combustion of hydrocarbons such as pentane ( c5 h12 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.(a) write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. (b) suppose 0.350 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 degree c. calculate the volume of carbon dioxide gas that is produced. be sure your answer has the correct number of significant digits.
Answers: 2

Chemistry, 22.06.2019 22:00, genyjoannerubiera
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

You know the right answer?
18. Predict the products for this single replacement reaction.
Cuso, + ALS
AL(S03)2 + Cu
AL(S03)2 + Cu
Questions in other subjects:




Mathematics, 16.12.2020 21:30








