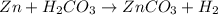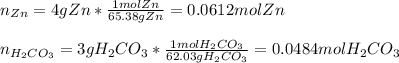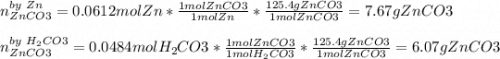Chemistry, 22.01.2021 08:30 davidtemple
1. Calculate the mass of the product, if you started with 4 g of Zn and 3 g of H2CO3.
Zn + H2CO3
ZnCO2 + H2 * I’ll cash app for the right answer plus explanation”


Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, NatalieKnows
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 19:30, xxaurorabluexx
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 22.06.2019 20:30, ShahinF7536
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
1. Calculate the mass of the product, if you started with 4 g of Zn and 3 g of H2CO3.
Zn + H2CO3
Questions in other subjects:



Social Studies, 20.07.2019 20:00


Social Studies, 20.07.2019 20:00

History, 20.07.2019 20:00



Mathematics, 20.07.2019 20:00