
Chemistry, 22.01.2021 06:00 willoughbysierra
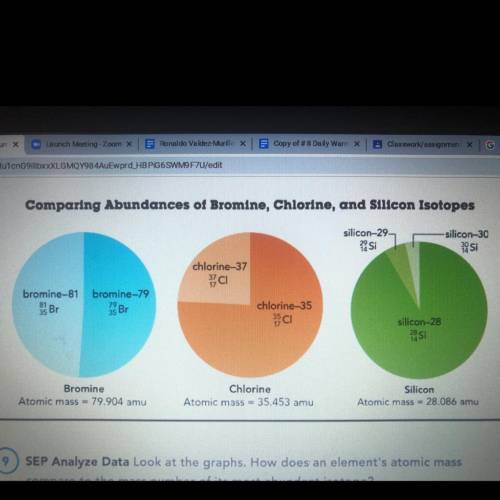
Look at the graphs. How does an element's atomic mass compare to the mass number of its most abundant isotope?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:30, xlebrny7831
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2



Chemistry, 23.06.2019 06:50, isabellainksow87vn
The student repeated the experiment using a higher concentration of acid. the same volume of acid and the same mass of magnesium ribbon were used. what volume of hydrogen gas would have been produced after 60 seconds?
Answers: 1
You know the right answer?
Look at the graphs. How does an element's atomic mass compare to the mass number of its most abundan...
Questions in other subjects:

Mathematics, 12.11.2020 19:10


Mathematics, 12.11.2020 19:10



Social Studies, 12.11.2020 19:10



Biology, 12.11.2020 19:10




