
1. Write the balanced chemical equation for the reaction you are performing.
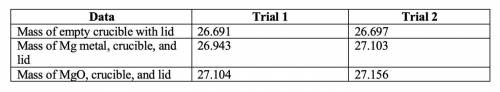
2. Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass of Mg, crucible, and lid (row 2 in the chart) to find the mass of magnesium for each trial.
• Trial 1: 0.252
• Trial 2: 0.406
3. Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass of MgO, crucible, and lid (row 3 in the chart) to find the mass of magnesium oxide for each trial. This is the actual yield of magnesium oxide for each trial.
• Trial 1: 0.413
• Trial 2: 0.459
4. Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for each trial.
• Trial 1:
• Trial 2:
5. Determine the percent yield of MgO for your experiment for each trial.
• Trial 1:
• Trial 2:
6. Determine the average percent yield of MgO for the two trials.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, annafellows
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1


Chemistry, 22.06.2019 10:40, trinityanne1738
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 17:20, phanuel642
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
You know the right answer?
1. Write the balanced chemical equation for the reaction you are performing.
2. Subtract the mass o...
Questions in other subjects:

Mathematics, 03.02.2020 18:48

Arts, 03.02.2020 18:48



English, 03.02.2020 18:48





Mathematics, 03.02.2020 18:49



