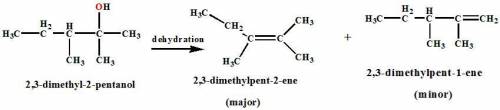
Chemistry, 21.01.2021 23:00 enrique764058
Identify the most common reaction conditions for the dehydration of 2,3-dimethyl-2-pentanol. A line-angle structure of 2,3-dimethyl-2-pentanol shows a chain of the following sequence:
a. A line terminus, a segment of three vertices, and a line terminus.
b. A CH3 group is attached to the first (from left to right) and the second vertices.
c. An OH group is attached to the first vertex.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 11:40, arlabbe0606
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3


Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
Identify the most common reaction conditions for the dehydration of 2,3-dimethyl-2-pentanol. A line-...
Questions in other subjects:

Mathematics, 29.10.2020 06:00

Mathematics, 29.10.2020 06:00


Mathematics, 29.10.2020 06:00

Business, 29.10.2020 06:00



Mathematics, 29.10.2020 06:00