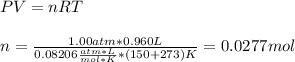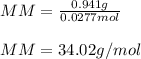Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, nothingworksoutforme
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

You know the right answer?
A sample of an unknown compound is vaporized at 150.°C . The gas produced has a volume of 960.mL at...
Questions in other subjects:

Social Studies, 16.09.2020 19:01

Biology, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Social Studies, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01