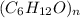Chemistry, 21.01.2021 22:10 allyssaharrisooy50au
A 2.912 g sample of a compounds containing only C, H, and O was completely oxidized in a reaction that yielded 3.123 g of water and 7.691 g of carbon dioxide. Determine the empirical formula and molecular formula of the compound if it has a molar mass of 100.1 g/mol.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, jmanrules200
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1



Chemistry, 23.06.2019 01:00, williedenmark42
What is the most common form of matter in the universe
Answers: 2
You know the right answer?
A 2.912 g sample of a compounds containing only C, H, and O was completely oxidized in a reaction th...
Questions in other subjects:

Mathematics, 20.04.2021 01:30

Mathematics, 20.04.2021 01:30

Mathematics, 20.04.2021 01:30


Health, 20.04.2021 01:30


English, 20.04.2021 01:30



Mathematics, 20.04.2021 01:30