

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, reeceslife481
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 02:30, caeyanij
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 14:00, BrandyLeach01
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
You know the right answer?
A student titrates 0.2087 g of an unknown diprotic acid with 0.1224 M sodium hydroxide. It takes 32....
Questions in other subjects:




Mathematics, 08.02.2021 22:30

Mathematics, 08.02.2021 22:30


Mathematics, 08.02.2021 22:30

Social Studies, 08.02.2021 22:30


English, 08.02.2021 22:30

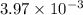 moles.
moles. 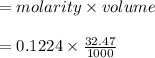
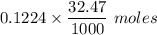
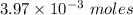 .
.

