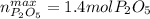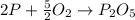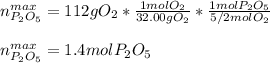Chemistry, 21.01.2021 21:40 alyxxboothe
What is the maximum amount in moles of P2O5P2O5 that can theoretically be made from 112 gg of O2O2 and excess phosphorus

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, DarcieMATHlin2589
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 14:00, Killion2022
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 20:00, Chynadoll94
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 23.06.2019 08:00, Robinlynn228
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 1
You know the right answer?
What is the maximum amount in moles of P2O5P2O5 that can theoretically be made from 112 gg of O2O2 a...
Questions in other subjects:

Mathematics, 03.06.2021 22:20


Mathematics, 03.06.2021 22:20

Mathematics, 03.06.2021 22:20

Biology, 03.06.2021 22:20

Biology, 03.06.2021 22:20

Biology, 03.06.2021 22:20

Mathematics, 03.06.2021 22:20


Mathematics, 03.06.2021 22:20