
Chemistry, 21.01.2021 15:40 cxttiemsp021
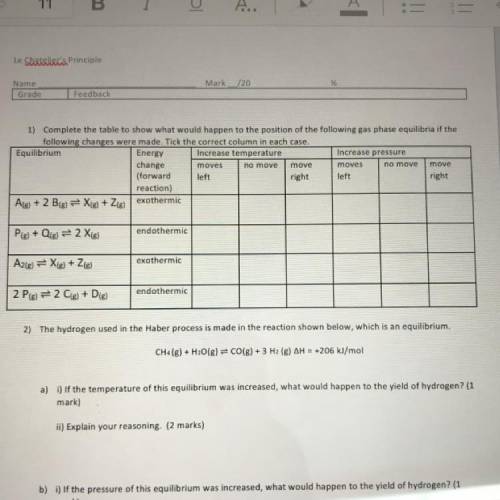
1) Complete the table to show what would happen to the position of the following gas phase equilibria if the
following changes were made. Tick the correct column in each case.
Equilibrium
Energy Increase temperature
Increase pressure
change
moves
moves
move
(forward left
right left
right
reaction)
Ale) + 2 B(g) = X(g) + Zig) exothermic
endothermic
Ple) + Qig) = 2 Xig)
exothermic
A2(g) = X(g) + 2(g)
endothermic
2 Pig) = 2 C(s) + D(g)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, elizabethprasad2
How many grams of n2h4 will be consumed by 23 g of n2o4
Answers: 1


Chemistry, 22.06.2019 13:00, carlinryan
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

You know the right answer?
1) Complete the table to show what would happen to the position of the following gas phase equilibri...
Questions in other subjects:

Mathematics, 01.03.2021 17:50

Mathematics, 01.03.2021 17:50


Mathematics, 01.03.2021 17:50



Mathematics, 01.03.2021 17:50

Mathematics, 01.03.2021 17:50

Mathematics, 01.03.2021 17:50



