
Chemistry, 21.01.2021 08:50 raymond5799
8. For each of the following pairs of atoms, highlight the atom that will attract shared electrons more
strongly.
a. Carbon or
Chlorine
b. Rubidium or Bromine
C. lodine or Indium
d. Silver
Sulfur
e. Arsenic
or
Sodium
f. Hydrogen or Selenium
or
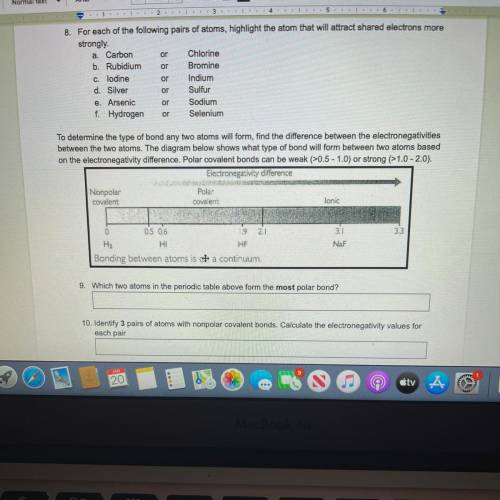
To determine the type of bond any two atoms will form, find the difference between the electronegativities
between the two atoms. The diagram below shows what type of bond will form between two atoms based
on the electronegativity difference. Polar covalent bonds can be weak (>0.5 - 1.0) or strong (>1.0-2.0).
Electronegativity difference
Nonpolar
Polas
covalent
covalent
lonic
0
2
3.3
0.5 0.6
9
H2
HI
HF
Bonding between atoms is a continuum.
3.1
NaF
9. Which two atoms in the periodic table above form the most polar bond?
10. Identify 3 pairs of atoms with nonpolar covalent bonds. Calculate the electronegativity values
each pair

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, Brookwiggington8814
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1


Chemistry, 22.06.2019 17:10, glitterpanda2468
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 20:30, dinapaul424
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
You know the right answer?
8. For each of the following pairs of atoms, highlight the atom that will attract shared electrons m...
Questions in other subjects:

English, 24.02.2021 03:20

Mathematics, 24.02.2021 03:20

Mathematics, 24.02.2021 03:20


Geography, 24.02.2021 03:20


Mathematics, 24.02.2021 03:20

Computers and Technology, 24.02.2021 03:20


Mathematics, 24.02.2021 03:20



