energy than cesium.

Chemistry, 21.01.2021 03:40 YARETZYVENCES2144
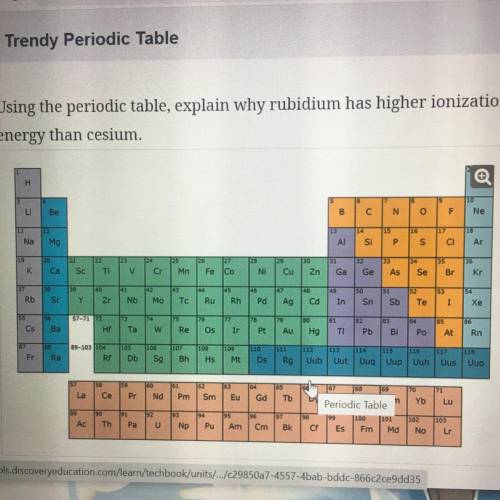
Using the periodic table, explain why rubidium has higher ionization
energy than cesium.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, willcohen42
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 23:30, treylartigue
The appropriate concentration for an iodine sanitizer is
Answers: 1
You know the right answer?
Using the periodic table, explain why rubidium has higher ionization
energy than cesium.
energy than cesium.
Questions in other subjects:



Mathematics, 08.01.2021 19:00


Mathematics, 08.01.2021 19:00


Health, 08.01.2021 19:00


Mathematics, 08.01.2021 19:00

Mathematics, 08.01.2021 19:00