
--What do you have to do to the coefficients of equation I below to get to equation II?
i) 2 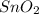 + 4
+ 4 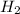 --> 2 Sn + 4
--> 2 Sn + 4 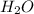
ii) 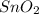 + 2
+ 2 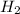 --> Sn + 2
--> Sn + 2 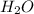
--Can you divide equation II by another factor and still have it be correct? Why or why not?
--In a complete sentence, write down a method you could use to determine if an equation is written in the correct way.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:00, lucyamine0
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 23.06.2019 01:00, kwarwick0915
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 01:30, kenldykido2300
Adirect relationship can be represented by: a curve a pie chart
Answers: 2

Chemistry, 23.06.2019 01:30, sheldonwaid4278
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
--What do you have to do to the coefficients of equation I below to get to equation II?
i) 2 + 4...
Questions in other subjects:


Mathematics, 16.11.2020 01:00

Mathematics, 16.11.2020 01:00

Physics, 16.11.2020 01:00

Mathematics, 16.11.2020 01:00


Social Studies, 16.11.2020 01:00





