

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:00, notearslefttocry14
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 23.06.2019 03:30, vaehcollier
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1

Chemistry, 23.06.2019 03:50, timothymoles
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1
You know the right answer?
If 45.00 g of precipitate is formed from the reaction of 0.100 mol/L
HCL(aq) and 0.124 mol/L AgNO3(...
Questions in other subjects:



Biology, 05.10.2019 03:00

Mathematics, 05.10.2019 03:00

English, 05.10.2019 03:00


Mathematics, 05.10.2019 03:00

English, 05.10.2019 03:00


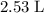 (rounded to three significant figures) assuming that
(rounded to three significant figures) assuming that 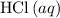 is in excess.
is in excess.  and
and 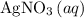 precipitate,
precipitate, 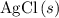 (the said precipitate) and
(the said precipitate) and 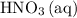 are produced:
are produced: (verify that this equation is indeed balanced.)
(verify that this equation is indeed balanced.)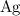 and
and 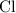 on a modern periodic table:
on a modern periodic table: .
.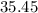 .
.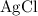 :
: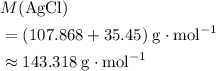 .
. of this compound:
of this compound: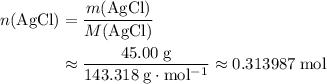 .
.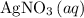 and
and 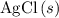 are both one.
are both one. 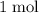 of
of 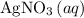 formula units to produce
formula units to produce 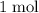 of
of 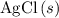 formula units.
formula units. 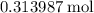 of
of 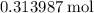 of
of 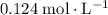 :
: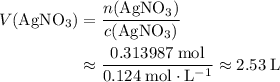 .
.

