
Chemistry, 20.01.2021 08:00 DuckieTime
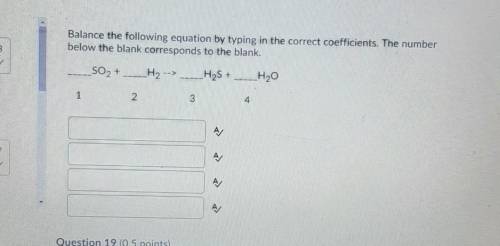
Question 1: Balance the following equation by typing in the correct coefficients. The number below the blank corresponds to the blank.
___SO2 +_H2 --> _H2S + _H2O
Question 2: What type of reaction results in the release of heat and light?
A) Endothermic
B) Combustion
C) Synthesis
Question 3: The generic formula AB -> A + B applies to what type of reaction.
A) Synthesis
B) Combustion
C) Single Displacement
D) Decomposition

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, tifftifftiff5069
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 06:00, girly37
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 09:10, aleilyg2005
Select the correct answer from each drop-down menu. describe what happens to a carbon-11 atom when it undergoes positron emission. the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3
You know the right answer?
Question 1: Balance the following equation by typing in the correct coefficients. The number below t...
Questions in other subjects:

Mathematics, 30.09.2020 05:01





Mathematics, 30.09.2020 05:01






