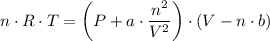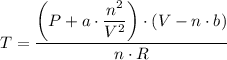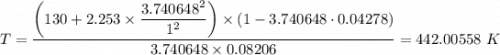Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1


Chemistry, 22.06.2019 22:30, kiera2599
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1

Chemistry, 23.06.2019 10:30, fatheadd2007
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
When 60.0 g methane (CH4) is placed in a 1.00-L vessel, the pressure is measured to be 130 atm. Calc...
Questions in other subjects:





Chemistry, 24.06.2019 02:00



Biology, 24.06.2019 02:00


Biology, 24.06.2019 02:00