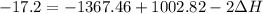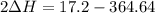Chemistry, 19.01.2021 19:40 supergraciepie
A scientist measures the standard enthalpy change for the following reaction to be -17.2 kJ : Ca(OH)2(aq) 2 HCl(aq)CaCl2(s) 2 H2O(l) Based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation of HCl(aq) is kJ/mol.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, jordan5778
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3


Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2
You know the right answer?
A scientist measures the standard enthalpy change for the following reaction to be -17.2 kJ : Ca(OH)...
Questions in other subjects:


Social Studies, 19.05.2021 16:40


Mathematics, 19.05.2021 16:40



Social Studies, 19.05.2021 16:40

Mathematics, 19.05.2021 16:40

Mathematics, 19.05.2021 16:40

Mathematics, 19.05.2021 16:40

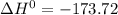 kJ/mol
kJ/mol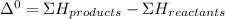
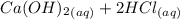 →
→ 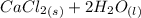
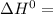 -1002.82 kJ/mol
-1002.82 kJ/mol![-17.2=[-795.8+2(285.85)]-[-1002.82+2\Delta H]](/tpl/images/1045/5683/27b56.png)