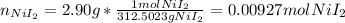Suppose 2.90g of nickel(II) iodide is dissolved in 150ml of a 0.70M aqueous solution of potassium carbonate. Calculate the final molarity of iodide anion in the solution. You can assume the volume of the solution doesn't change when the nickel(II) iodide is dissolved in it. Round your answer to 3 significant digits.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, Chynadoll94
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 22.06.2019 23:30, hcllxxhhlpcj
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 07:00, jaydenboi604
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample?
Answers: 1
You know the right answer?
Suppose 2.90g of nickel(II) iodide is dissolved in 150ml of a 0.70M aqueous solution of potassium ca...
Questions in other subjects:

Mathematics, 18.11.2020 01:00





Mathematics, 18.11.2020 01:00

Mathematics, 18.11.2020 01:00


Mathematics, 18.11.2020 01:00