Which of the following is true?
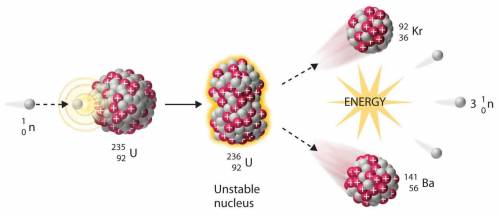
The final reaction produces two, much smaller radioisotopes.
...

Chemistry, 19.01.2021 18:00 juliangarcia0002
Which of the following is true?
The final reaction produces two, much smaller radioisotopes.
The final reaction produces two, much smaller radioisotopes.
The overall reaction produces three neutrons for every neutron used.
The overall reaction produces three neutrons for every neutron used.
The breakup of the Uranium-236 produces an extremely large amount of energy.
The breakup of the Uranium-236 produces an extremely large amount of energy.
The bombardment of Uranium-236 produces a very unstable intermediate product.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, sandersmakaylaovq5vu
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 21.06.2019 22:00, carlybeavers50
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 21.06.2019 23:00, jasmineharris121
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 00:30, thatonestudent2271
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 05.03.2021 01:00



Mathematics, 05.03.2021 01:00

History, 05.03.2021 01:00

Mathematics, 05.03.2021 01:00

Mathematics, 05.03.2021 01:00

Engineering, 05.03.2021 01:00

Mathematics, 05.03.2021 01:00

Biology, 05.03.2021 01:00



