
Chemistry, 19.01.2021 03:20 bellbradshaw16
Aqueous solutions of Na2CO3 and Ca(NO3)2, 0.10 M each, are combined. A white precipitate is observed in the container after mixing. The precipitate is filtered and carefully rinsed with distilled water to remove other ions. A sample of the precipitate is added to 100 mL of 0.1 M NaCl. A second sample of the precipitate is then added to 100 mL of 0.1 M HCl. What would be observed in each case

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:50, josegalvan123jg69
Which statement is a reason to support population regulation? a) it is unethical for us to control birth control rates b) humans have the freedom to produce as many children as desired c) the gap between the rich and poor has been narrowing since 1960 d) billions more people on the earth will intensify many environmental and social problems
Answers: 1


Chemistry, 21.06.2019 21:40, MathChic68
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3
You know the right answer?
Aqueous solutions of Na2CO3 and Ca(NO3)2, 0.10 M each, are combined. A white precipitate is observed...
Questions in other subjects:




English, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30




Physics, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30

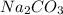 and
and  , 0.10 M each, are combined. A white precipitate is observed in the container after mixing. he precipitate is filtered andcarefully rinsed with distilled water to remove other ions. A sample of the precipitate is added to 100 mL of 0.1 M NaCl. A second sample of the precipitate is then added to 100 mL of 0.1 M HCl. What would be observed in each case?
, 0.10 M each, are combined. A white precipitate is observed in the container after mixing. he precipitate is filtered andcarefully rinsed with distilled water to remove other ions. A sample of the precipitate is added to 100 mL of 0.1 M NaCl. A second sample of the precipitate is then added to 100 mL of 0.1 M HCl. What would be observed in each case?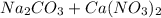 →
→ 
 ), which, as it is insoluble in water, precipitates as a solid of the color white. This process is Precipitation and this reaction is a Precipitation Reaction.
), which, as it is insoluble in water, precipitates as a solid of the color white. This process is Precipitation and this reaction is a Precipitation Reaction.  →
→ 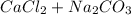
 →
→ 


