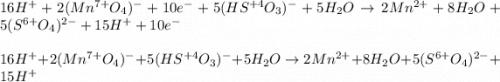Chemistry, 19.01.2021 01:10 lankfordcrystal81
Balance the following redox equations. All occur in acid solution. (Type your answer using the format CO2 for CO2 and [NH4] for NH4 . Use the lowest possible coefficients.) (a) MnO4-(aq) (aq) HSO3-(aq) Mn2 (aq) (l) SO42-(aq)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, giusto1894
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 10:20, blondielocks2002
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3
You know the right answer?
Balance the following redox equations. All occur in acid solution. (Type your answer using the forma...
Questions in other subjects:

Physics, 12.05.2021 17:00

Spanish, 12.05.2021 17:00

Computers and Technology, 12.05.2021 17:00


English, 12.05.2021 17:00

Mathematics, 12.05.2021 17:00


English, 12.05.2021 17:00


Mathematics, 12.05.2021 17:00

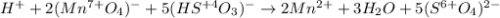
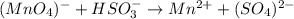
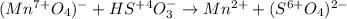
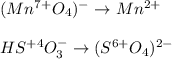
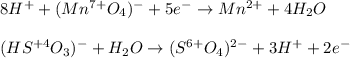
![2(8H^++(Mn^{7+}O_4)^-+5e^-\rightarrow Mn^{2+}+4H_2O)\\\\5[(HS^{+4}O_3)^-+H_2O\rightarrow (S^{6+}O_4)^{2-}+3H^++2e^-]\\\\\\16H^++2(Mn^{7+}O_4)^-+10e^-\rightarrow 2Mn^{2+}+8H_2O\\\\5(HS^{+4}O_3)^-+5H_2O\rightarrow 5(S^{6+}O_4)^{2-}+15H^++10e^-](/tpl/images/1043/9796/c0cd7.png)