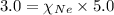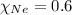Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:50, mckinleesmomp6qj1e
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 08:00, tchase0616
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 09:40, gonzaleze18
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 23:00, edgar504xx
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
A 250 mL flask initially contains argon at a pressure of 2.0 atm. Neon is added to the flask until t...
Questions in other subjects:

Mathematics, 30.01.2021 08:10

History, 30.01.2021 08:10

Physics, 30.01.2021 08:10

Mathematics, 30.01.2021 08:10




Mathematics, 30.01.2021 08:10


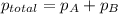
 =total pressure of gases = 5.0 atm
=total pressure of gases = 5.0 atm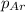 = partial pressure of argon = 2.0 atm
= partial pressure of argon = 2.0 atm
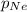 = partial pressure of Neon = ? atm
= partial pressure of Neon = ? atm 


 = mole fraction of Neon
= mole fraction of Neon