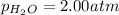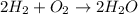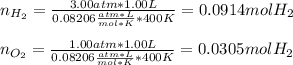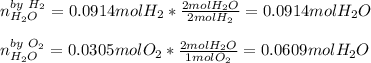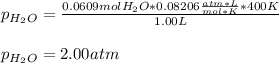Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:30, needhelpasap8957
Why is the bottom layer of a trophic pyrimid the
Answers: 2

Chemistry, 23.06.2019 08:30, vett072804
Benzonitrile (c6h5cn) is reduced to two different products depending on the reducing agent used. treatment with lithium aluminum hydride followed by water forms k, which has a molecular ion in its mass spectrum at 107 and the following ir absorptions: 3373, 3290, 3062, 2920, and 1600 cm-1. treatment with a milder reducing agent forms l, which has a molecular ion in its mass spectrum at 106 and the following ir absorptions: 3086, 2850, 2820, 2736, 1703, and 1600 cm-1. l shows fragments in its mass spectrum at m/z = 105 and 77. propose structures for k and l and choose an explanation for how this could be concluded.
Answers: 3
You know the right answer?
A gas mixture contains 3.00 atm of H2 and 1.00 atm of O2 in a 1.00 L vessel at 400K. If the mixture...
Questions in other subjects:

Mathematics, 16.04.2020 03:33









Mathematics, 16.04.2020 03:33