Chemistry, 18.01.2021 21:40 lkarroum3733
Calculate the volume in mL of 1.043 M acetic acid (CH3COOH) that is needed to prepare 50.0 mL of 0.10 M acetic acid. Show your work and give your answer to the correct number of significant figures.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, bbyniah123
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 12:30, quantamagic
Word equation for k(s)+h2o(l) yield koh(aq) + h2(g)
Answers: 1

You know the right answer?
Calculate the volume in mL of 1.043 M acetic acid (CH3COOH) that is needed to prepare 50.0 mL of 0.1...
Questions in other subjects:


Biology, 17.02.2021 22:00

Chemistry, 17.02.2021 22:00

Mathematics, 17.02.2021 22:00

Mathematics, 17.02.2021 22:00

Mathematics, 17.02.2021 22:00

Biology, 17.02.2021 22:00



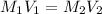
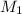 = molarity of stock
= molarity of stock 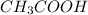 solution = 1.043 M
solution = 1.043 M
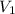 = volume of stock
= volume of stock