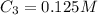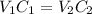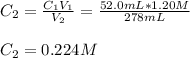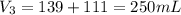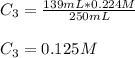Chemistry, 18.01.2021 21:10 tylermorehead1
A 52.0 mL portion of a 1.20 M solution is diluted to a total volume of 278 mL. A 139 mL portion of that solution is diluted by adding 111 mL of water. What is the final concentration

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2


Chemistry, 22.06.2019 20:30, lexibyrd120
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

You know the right answer?
A 52.0 mL portion of a 1.20 M solution is diluted to a total volume of 278 mL. A 139 mL portion of t...
Questions in other subjects:

Biology, 12.12.2020 22:00

Arts, 12.12.2020 22:00


Mathematics, 12.12.2020 22:00

Mathematics, 12.12.2020 22:00



History, 12.12.2020 22:00

Mathematics, 12.12.2020 22:00