
Chemistry, 18.01.2021 16:50 dragongacha777
PLEASE HELP ME
1. What is the molality of a solution made up of 43.6 mol of CaCl2 dissolved by 13.5 kg of water?
2. How many moles of Na2CO3 are required to create 9.54 liters of a 3.4 M solution?
3. Which of these actions can result in decreasing the molarity of a solution?
Select all that apply.
A
adding solute
B
adding solvent
C
removing solute
D
removing solvent
Thanks Y'all:)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, shradhwaip2426
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 23.06.2019 01:30, yarrito20011307
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

You know the right answer?
PLEASE HELP ME
1. What is the molality of a solution made up of 43.6 mol of CaCl2 dissolved by 13.5...
Questions in other subjects:



Mathematics, 07.05.2021 19:10

History, 07.05.2021 19:10

English, 07.05.2021 19:10

Mathematics, 07.05.2021 19:10




Mathematics, 07.05.2021 19:10

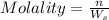
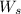 = weight of solvent
= weight of solvent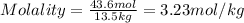
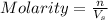
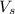 = volume of solution in L
= volume of solution in L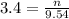
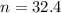
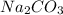 is 32.4
is 32.4 

