
Chemistry, 18.01.2021 16:30 21teunissen149
A group 2 metal carbonate has a mass of 84 g/mol. Identify the group 2 metal X using its chemical formula. Please show your working out. number of moles in 0.3g of calcium carbonate.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 09:00, phebusadrian01
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 09:00, triddi666
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
You know the right answer?
A group 2 metal carbonate has a mass of 84 g/mol. Identify the group 2 metal X using its chemical fo...
Questions in other subjects:


French, 18.12.2020 18:50

Chemistry, 18.12.2020 18:50


Mathematics, 18.12.2020 18:50

Mathematics, 18.12.2020 18:50

English, 18.12.2020 18:50

Mathematics, 18.12.2020 18:50


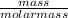
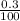 = 0.003mole
= 0.003mole

