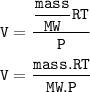Chemistry, 16.01.2021 18:50 Quidlord03
.25 g of each of the following gasses are taken at 27°C and 600 mm
Hg pressure. Which of these will have the least volume?
(Molar mass (g/mole): HCI = 36.5, HBr = 81, HI = 128, HF = 20)
(R=0.082 Latm/K. mole)
A. HC1
B. HBr
C. HI
D. HF

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, amanda2003teddy
Litmus paper is made from water-soluble dyes which are extracted from lichens. this paper is used as an acid-base indicator. which of these common household substances would turn blue litmus paper red? a) bleach b) lye c) soap d) vinegar
Answers: 3

Chemistry, 22.06.2019 00:00, lakenyagillard79
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 01:00, AIhunter2884
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2
You know the right answer?
.25 g of each of the following gasses are taken at 27°C and 600 mm
Hg pressure. Which of these will...
Questions in other subjects:





Mathematics, 11.03.2020 06:28


Mathematics, 11.03.2020 06:28