
Chemistry, 16.01.2021 14:40 jakeyywashere
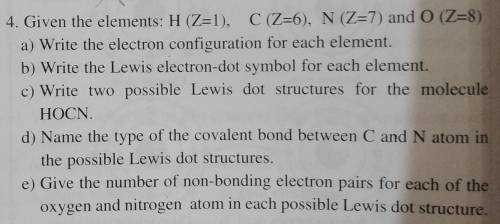
4. Given the elements: H (Z=1), C (Z=6), N (Z=7) and 0 (Z=8)
a) Write the electron configuration for each element.
b) Write the Lewis electron-dot symbol for each element.
c) Write two possible Lewis dot structures for the molecule
HOCN.
d) Name the type of the covalent bond between C and N atom in
the possible Lewis dot structures.
e) Give the number of non-bonding electron pairs for each of the
oxygen and nitrogen atom in each possible Lewis dot structure

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, isaiahrodriguezsm17
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 19:00, elizabethajih99
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 22.06.2019 20:40, larkinc2946
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1
You know the right answer?
4. Given the elements: H (Z=1), C (Z=6), N (Z=7) and 0 (Z=8)
a) Write the electron configuration fo...
Questions in other subjects:


Biology, 17.02.2022 19:00

Geography, 17.02.2022 19:00

Social Studies, 17.02.2022 19:00


Mathematics, 17.02.2022 19:00


Mathematics, 17.02.2022 19:00




