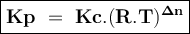Chemistry, 16.01.2021 14:00 savannah647
Kp/Kc for reaction for the equilibrium, A(g) ⇌ C(g)+B(g), is .
(Kc is the equilibrium constant in terms of concentrations, Kp is the equilibrium constant in terms of pressures, R is the gas constant, T is the temperature)
Select one:
(RT)2
(RT)-1
(RT)-2
(RT)-1.5
RT

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2


Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

You know the right answer?
Kp/Kc for reaction for the equilibrium, A(g) ⇌ C(g)+B(g), is .
(Kc is the equilibrium constant in t...
Questions in other subjects:

Mathematics, 29.10.2019 21:31

Computers and Technology, 29.10.2019 21:31

Health, 29.10.2019 21:31


Mathematics, 29.10.2019 21:31





![\large {\boxed {\bold {Kc ~ = ~ \frac {[C] ^ m [D] ^ n} {[A] ^ p [B] ^ q}}}}](/tpl/images/1041/5418/55d71.png)
![\large {\boxed {\bold {Kp ~ = ~ \frac {[pC] ^ m [pD] ^ n} {[pA] ^ p [pB] ^ q}}}}](/tpl/images/1041/5418/1041d.png)