
Chemistry, 15.01.2021 14:00 jacobbrandon2002
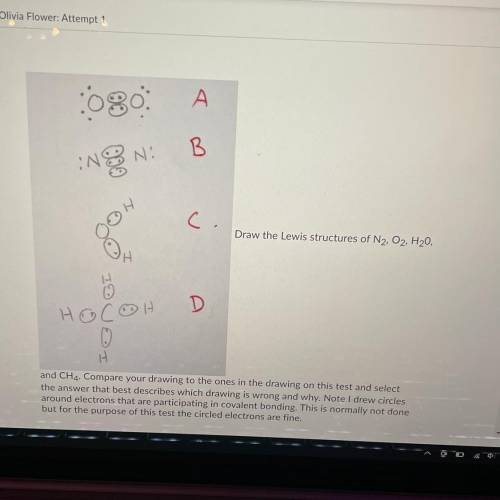
Draw the Lewis structures of N2, O2, H20, and CH4
Compare your drawing to the ones in the drawing on this test and select the answer that best describes which drawing is wrong and why.
A: O2 is wrong because it shows the electrons at a 45 degree angle to the Oxygen atoms
B: N2 is wrong because it shows a triple bond
C: H2O is wrong because it is missing 4 valence electrons
D: CH4 is wrong because the bonds are supposed to be bent at 109.5 degrees

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, sannai0415
Zinc + lead(ii) nitrate yield zinc nitrate + leadwhat's the chemical equation for this?
Answers: 1



Chemistry, 22.06.2019 21:30, leenzazou587
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
Draw the Lewis structures of N2, O2, H20, and CH4
Compare your drawing to the ones in the drawing o...
Questions in other subjects:

Mathematics, 25.11.2021 14:00

Mathematics, 25.11.2021 14:00




Chemistry, 25.11.2021 14:00






