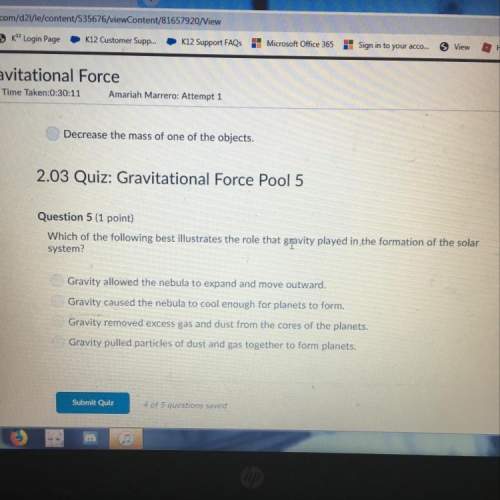
Chemistry, 14.01.2021 20:10 ayahabdulhaqq2
If you are struggling talk to me

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 03:00, winterblanco
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3


Chemistry, 23.06.2019 10:30, EstherAbuwaah
Identify the limiting reactant when 9.65-g h2so4 reacts with 6.10-g of naoh. the equation is h2s04 + 2naoh = 2h2o + na2so4• what is the theoretical yield of na2so4, in grams? • how much of the excess reagent will remain after the reaction has been completed? • if 10.5-g of na2so4 are actually recovered experimentally, what is the percent yield?
Answers: 3
You know the right answer?
If you are struggling talk to me...
Questions in other subjects:

Mathematics, 09.12.2021 08:00

Chemistry, 09.12.2021 08:00




Chemistry, 09.12.2021 08:00

Mathematics, 09.12.2021 08:00

Mathematics, 09.12.2021 08:00

Mathematics, 09.12.2021 08:00