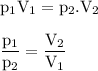Chemistry, 14.01.2021 18:00 cxttiemsp021
If a gas had a volume of 6.7 L and started at STP, what would the new pressure be if the volume ended at 1.5 L?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:40, monkey2865
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 21.06.2019 19:00, eweqwoewoji
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 14:30, Dreynolds1667
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
If a gas had a volume of 6.7 L and started at STP, what would the new pressure be
if the volume end...
Questions in other subjects:

Mathematics, 25.07.2019 10:00


History, 25.07.2019 10:00


English, 25.07.2019 10:00


Mathematics, 25.07.2019 10:00



History, 25.07.2019 10:00