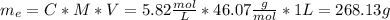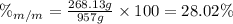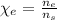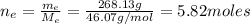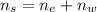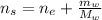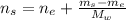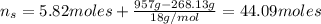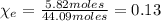Chemistry, 14.01.2021 17:00 aidengalvin20
An aqueous solution of ethanol, CH3CH2OH, has a concentration of 5.82 mol/L and has a density of 0.957 g/mL. What are the mass percent and mole fraction of CH3CH2OH in this solution

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, krharris
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 15:00, NatalieKnows
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 17:00, princessakosua2
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
You know the right answer?
An aqueous solution of ethanol, CH3CH2OH, has a concentration of 5.82 mol/L and has a density of 0.9...
Questions in other subjects:


History, 02.02.2021 02:50



Mathematics, 02.02.2021 02:50


Mathematics, 02.02.2021 02:50



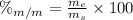
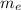 is the mass of ethanol
is the mass of ethanol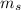 is the mass of the solution
is the mass of the solution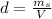
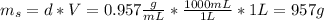
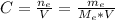
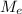 : is the molar mass of ethanol = 46.07 g/mol
: is the molar mass of ethanol = 46.07 g/mol 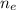 : is the number of moles of ethanol = m/M
: is the number of moles of ethanol = m/M