Chemistry, 14.01.2021 16:40 thanks5640
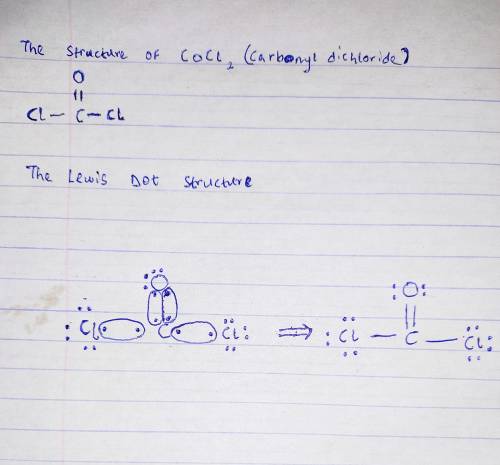
Draw the Lewis structure for the compound with the formula COCl2. Use lines to show bonding electrons. Draw the molecule by placing atoms on the canvas and connecting them with bonds. Include all lone pairs of electrons.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, pettygirl13
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3


Chemistry, 22.06.2019 15:10, kolbehoneyman
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion. what is the frequency f of oscillation?
Answers: 2
You know the right answer?
Draw the Lewis structure for the compound with the formula COCl2. Use lines to show bonding electron...
Questions in other subjects:

Mathematics, 03.10.2019 04:30

Mathematics, 03.10.2019 04:30

English, 03.10.2019 04:30