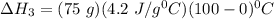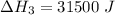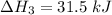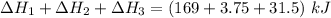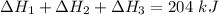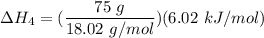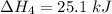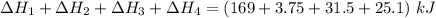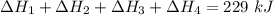Chemistry, 14.01.2021 16:40 herringalyssa
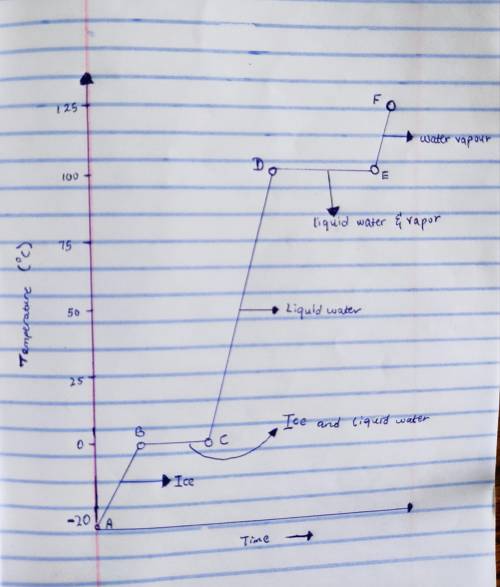
Consider a 75.0-g sample of H2O(g) at 1258C. What phase or phases are present when 215 kJ of energy is removed from this sample

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:50, limelight11
Which statement describes how phase changes can be diagrammed as a substance is heated? the phase is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the phase is on the x-axis. the time is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the time is on the x-axis.
Answers: 1

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 15:00, Zagorodniypolina5
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 23:00, brianfranklin17
What is the correct lewis dot structure for arsenic?
Answers: 2
You know the right answer?
Consider a 75.0-g sample of H2O(g) at 1258C. What phase or phases are present when 215 kJ of energy...
Questions in other subjects:

Business, 07.07.2019 14:20

History, 07.07.2019 14:20

History, 07.07.2019 14:20


Chemistry, 07.07.2019 14:20



Chemistry, 07.07.2019 14:20

History, 07.07.2019 14:20

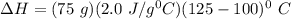
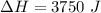
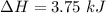
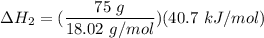
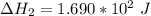
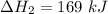
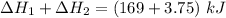
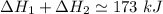 which is still lesser than 215 kJ
which is still lesser than 215 kJ